Solar cells, also known as photovoltaic cells, are getting very popular nowadays. People around the world are moving toward solar energy to save electric bills and the burning planet. But one common question that people may have in their minds is what are solar cells made of. A short and quick answer to the question is crystalline silicon (c-Si). Crystalline silicon is the crystalline form of silicon commonly used in semiconductors, including photovoltaic technology.
Most solar cells in the world mainly consist of crystalline silicon. However, not every solar cell is composed of silicon. There are materials too. Emerging solar technologies, especially second generation and third generation, are looking for different and better materials than predominant silicon. These new technologies face many challenges, for example, low efficiencies, and have not yet fully commercialized for residential and business consumers. Here, in the article, we have covered various materials used in different types of solar cells without going into too many technical aspects.
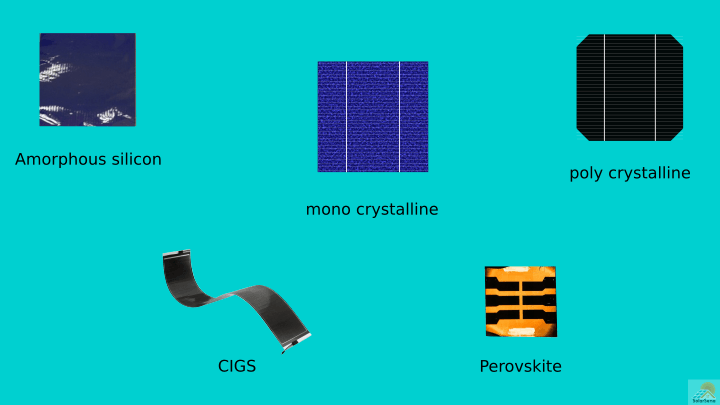
Crystalline silicon solar cells
Crystalline silicon solar cells are the first generation and traditional solar cells that we normally see on the roof or in the yard of someone’s home. Silicon is the base material of the electronic and semiconductor industry. Most of today’s electronics, such as mobile phones, laptops, have silicon microchips in them. The development of the use of silicon in photovoltaic technology began in the 1950s. Since then, it continues to remain dominant in the solar market.

The word “crystalline silicon” means silicon exists in the crystal form. Solar grade crystalline silicon is manufactured in industries by many chemical processes, like recrystallization. As of today, they prevail in the solar market because they give relatively higher efficiency than other emerging technologies. We can divide crystalline silicon into monocrystalline and polycrystalline.
Monocrystalline silicon
Monocrystalline silicon solar cells are made from a single crystal of silicon. They are more efficient but more expensive than polycrystalline. Further, they give a premium and aesthetic appearance. To make a monocrystalline silicon cell, pure silicon is molded into a cylindrical ingot and then cut into wafers. The corners of each cell are clipped, giving them an octagonal shape. Another physical distinguishing parameter is color. Monocrystalline silicon solar panels show black hue.
Since the entire unit is a homogeneous one-single piece, properties, like orientation, lattice parameter, and electronic properties, remain the same throughout the material. This increases the flowability of electrons in the material, making them more efficient.
Polycrystalline silicon
Polycrystalline silicon solar cells consist of polycrystalline silicon (poly c-Si). They are less expensive and easy to manufacture than monocrystalline. But they have a disadvantage of a low efficiency. Their manufacturing process is different from monocrystalline. In preparing this type, molten silicon is cooled and solidified to cast into a square block.

Unlike monocrystalline, the crystals of polycrystalline have random orientations and disuniformity inside. This gives hindrance to the flow of electrons, reducing the efficiency of the system. Solar panels made from polycrystalline silicon show bluish hue and are pretty common.
Thin-film solar cells
Thin-film solar cells are second-generation solar technology. They consist of one or more thin films of a photovoltaic material deposited on a substrate, such as a polymer, glass. The thickness of films in a range of nanometers, making them much thinner than crystalline silicon cells. Some of them are also flexible. Flexible thin-film solar cells have applications in building materials, like windows, curved roofs, and in solar vehicles. They are better than conventional c-Si cells in terms of material consumption.
There are three common types of solar cells based on the material of the construction. They include cadmium telluride, copper indium gallium selenide, and amorphous silicon.
Cadmium telluride
Cadmium telluride (CdTe) photovoltaic cells are prevalent in thin-film solar technology and a major rival to c-Si technology. It accounts for 5% of the total photovoltaic market and shares more than half of the thin-film production.

The two major limitations of this type are the toxicity of cadmium and the rarity of tellurium. Cadmium is dangerous if released from panels. And tellurium, unlike silicon, which is the second most common element, is a very rare element in the earth’s crust.
Copper indium gallium selenide
CIGS photovoltaic cells consist of four elements: copper, indium, gallium, and selenide. In some cases, gallium is absent, making it CIS cells. They have the highest efficiency in the thin-film market and held 2% of the total photovoltaic market in 2013.

In 2019, researchers had reported lab efficiency of more than 20%, which is equal to prominent c-Si cells. However, there are some uncertainties in the cost of technology.
Amorphous silicon
Amorphous silicon (a-Si) is non-crystalline silicon. It has an advantage over other thin-film materials since silicon is non-toxic and abundant in the earth’s crust. Amorphous silicon solar cells are manufactured by a process called chemical vapor deposition. The process involves silane (SiH4) and hydrogen and the deposition of silane on the glass as a substrate.

It is the most developed thin-film technology. Moreover, we can increase the efficiency of a-Si by pairing them with microcrystalline silicon to give tandem cells.
Third-generation solar cells
Third-generation photovoltaic cells are emerging and presently restricted within research laboratories. The concept of third-generation cells began to overcome the efficiency barrier of second- and first-generation solar cells. Nevertheless, none of these cells have crossed the efficiency of crystalline silicon cells. Dr. Scott Watkins, a solar cell scientist, holding a sheet of flexible organic solar cells [Credit: CSIRO/cc]
They use organic and/or inorganic compounds. Some of them are:
- Dye-sensitized solar cell (DSSC): DSSC contains an organic molecule dye that absorbs light supported by titanium dioxide nanoparticles.
- Organic solar cell (OSC): It uses organic materials—polymers and smaller organic molecules—to transfer charge carriers.
- Perovskite solar cell (PSC): It is a hybrid organic-inorganic solar cell. A common example is methylammonium lead trihalide.
- Copper zinc tin sulfide cell (CZTS): The crystals of CZTS consist of copper, zinc, tin, and sulfur.
- Quantum dot solar cell (QDS): It consists of tiny nanoparticles called quantum dots as a photovoltaic material.
Final thoughts
Solar technology is continually evolving and has shown no sign to stop since the 1950s. The selection and development of the right material are some major challenges. A lot of improvements are essential to break the efficient barrier. Based on the past progress, it is likely, for the coming years, crystalline solar cells will continue to prevail in the solar market. But in a very long period, third-generation solar cells will supplant the current technology.